
SL Paper 1
Which series shows the correct order of metallic bond strength from strongest to weakest?
A.
B.
C.
D.
Markscheme
D
Examiners report
A small majority of candidates could identify the relative strength of metallic bonding amongst group 1 and 2 metals.
What is the formula of the compound formed from Ca2+ and PO43−?
A. CaPO4
B. Ca3(PO4)2
C. Ca2(PO4)3
D. Ca(PO4)2
Markscheme
B
Examiners report
Which compound contains both ionic and covalent bonds?
A. CH3COONa
B. CH3COOH
C. K2O
D. CaCl2
Markscheme
A
Examiners report
What is the formula of magnesium nitride?
A. MgN
B. Mg2N3
C. Mg3N
D. Mg3N2
Markscheme
D
Examiners report
Which compound has the shortest C to O bond?
A. CH3CHO
B. CO
C. CO2
D. C2H5OC2H5
Markscheme
B
Examiners report
A substance has the following properties:
What is the most probable structure of this substance?
A. Network covalent
B. Polar covalent molecule
C. Ionic lattice
D. Metallic lattice
Markscheme
A
Examiners report
Which molecule has the weakest nitrogen to nitrogen bond?
A. N2
B. N2H2
C. N2H4
D.
Markscheme
C
Examiners report
A compound consists of the ions Ca2+ and PO43–. What are the name and formula of the compound?
Markscheme
D
Examiners report
Which combination corresponds to a strong metallic bond?
Markscheme
B
Examiners report
The concept of charge and ionic radii size on the strength of the bond was answered well, but had the lowest discriminatory index on the paper.
Which compound has the shortest C–N bond?
A. CH3NH2
B. (CH3)3CNH2
C. CH3CN
D. CH3CHNH
Markscheme
C
Examiners report
The following compounds have similar relative molecular masses. What is the order of increasing boiling point?
A. CH3CH2CH2OH < CH3CH2CHO < CH3COOH
B. CH3CH2CHO < CH3CH2CH2OH < CH3COOH
C. CH3CH2CHO < CH3COOH < CH3CH2CH2OH
D. CH3COOH < CH3CH2CHO < CH3CH2CH2OH
Markscheme
B
Examiners report
Which combination causes the strength of metallic bonding to increase?
Markscheme
D
Examiners report
A question about factors affecting the strength of metallic bonding answered correctly by 68 % of candidates. The distractors were almost equally chosen by the rest of the candidates.
Which combination would create the strongest ionic bond?
Markscheme
C
Examiners report
Which metal has the strongest metallic bond?
A. Li
B. Na
C. K
D. Rb
Markscheme
A
Examiners report
Which is correct for all solid ionic compounds?
A. High volatility
B. Poor electrical conductivity
C. Low melting point
D. Good solubility in water
Markscheme
B
Examiners report
Which alcohol is least soluble in water?
A. CH3OH
B. CH3CH2OH
C. CH3CH2CH2OH
D. CH3CH2CH2CH2OH
Markscheme
D
Examiners report
What are the predicted electron domain geometries around the carbon and both nitrogen atoms in urea, (NH2)2CO, applying VSEPR theory?
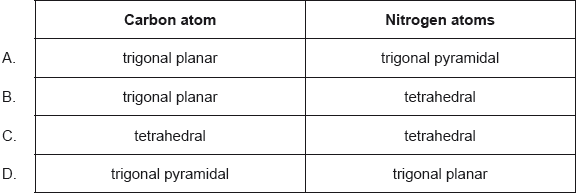
Markscheme
B
Examiners report
Which bonds cause the boiling point of water to be significantly greater than that of hydrogen sulfide?
A. London (dispersion)
B. Covalent
C. Ionic
D. Hydrogen
Markscheme
D
Examiners report
Which molecule is most polar?
A. CF4
B. CCl4
C. CHF3
D. CClF3
Markscheme
C
Examiners report
Question 11 was identified in the G2s as a tricky question, because students could have difficulty resolving the conflict between the greater polarity of the C-Cl bond compared with the C-H bond, and their contrast with the polarity of the C-F bond.) However, 56% of student did identify the correct answer of C.
Which molecule is most polar?
A.
B.
C.
D.
Markscheme
A
Examiners report
63% of the candidates could identify CHF3 as more polar than CClF3. A surprising number selected either CCl4 or CF4 as the most polar.
Which combination describes the sulfate(IV) ion, SO32– (also known as sulfite ion)?
Markscheme
D
Examiners report
What is the formula of ammonium phosphate?
A. (NH3)3PO4
B. (NH4)3PO4
C. (NH4)2PO4
D. (NH3)2PO3
Markscheme
B
Examiners report
What are the strongest intermolecular forces between molecules of propanone, CH3COCH3, in the liquid phase?
A. London (dispersion) forces
B. Covalent bonding
C. Hydrogen bonding
D. Dipole–dipole forces
Markscheme
D
Examiners report
Which describes an ionic compound?
Markscheme
B
Examiners report
Fairly well answered with the most widely held misconception being that ionic compounds conduct electricity in the solid state.
What is the main interaction between liquid CH4 molecules?
A. London (dispersion) forces
B. Dipole–dipole forces
C. Hydrogen bonding
D. Covalent bonding
Markscheme
A
Examiners report
56% of the candidates were able to identify London (dispersion) forces as the main interaction between liquid CH4 molecules. Covalent bonding was the most commonly chosen distractor. Good performance on this question correlated well with candidates who scored well overall.
What is the order of increasing boiling point?
A. CH3CH2CH2CH3 < CH3CH(OH)CH3 < CH3COCH3 < CH3CO2H
B. CH3CH2CH2CH3 < CH3COCH3 < CH3CH(OH)CH3 < CH3CO2H
C. CH3CO2H < CH3COCH3 < CH3CH(OH)CH3 < CH3CH2CH2CH3
D. CH3CH2CH2CH3 < CH3COCH3 < CH3CO2H < CH3CH(OH)CH3
Markscheme
B
Examiners report
Ranking compounds in order of increasing boiling point proved challenging. There existed a number of misconceptions based on the incorrect answers chosen being close to evenly distributed.
Which substance has a giant covalent structure?
Markscheme
D
Examiners report
How many bonding electrons are there in the urea molecule?
A. 8
B. 16
C. 20
D. 24
Markscheme
B
Examiners report
Which two atoms form the most polar bond?
A. C and F
B. C and Cl
C. Si and F
D. Si and Cl
Markscheme
C
Examiners report
Which of the following does not react with dilute HCl(aq)?
A. Na2CO3
B. Cu
C. Zn
D. CuO
Markscheme
B
Examiners report
Which compound has hydrogen bonds between its molecules?
A. CH4
B. CH4O
C. CH3Cl
D. CH2O
Markscheme
B
Examiners report
72 % of the candidates identified CH4O as having hydrogen bonds between its molecules. The most commonly chosen distractor was CH2O, the only other option containing oxygen.
How many lone pairs and bonding pairs of electrons surround the central chlorine atom in ClF2+?
Markscheme
D
Examiners report
The compounds shown below have similar relative molecular masses. What is the correct order of increasing boiling point?
A. CH3COOH < (CH3)2CO < (CH3)2CHOH
B. CH3COOH < (CH3)2CHOH < (CH3)2CO
C. (CH3)2CO < CH3COOH < (CH3)2CHOH
D. (CH3)2CO < (CH3)2CHOH < CH3COOH
Markscheme
D
Examiners report
What are the approximate bond angles and structure of crystalline SiO2?
Markscheme
B
Examiners report
What is the structure and bonding in SiO2 (s)?
Markscheme
A
Examiners report
Which series is in order of increasing boiling point?
A. CH2CH2CH3OH CH3COCH3 CH3CH2CH3
B. CH3CH2CH3 CH3COCH3 CH2CH2CH3OH
C. CH3COCH3 CH2CH2CH3OH CH3CH2CH3
D. CH3CH2CH3 CH2CH2CH3OH CH3COCH3
Markscheme
B
Examiners report
What is the order of increasing boiling point?
A. C4H10 < CH3COOH < CH3CH2CHO < CH3CH2CH2OH
B. C4H10 < CH3CH2CHO < CH3CH2CH2OH < CH3COOH
C. CH3COOH < CH3CH2CH2OH< CH3CH2CHO < C4H10
D. C4H10 < CH3CH2CH2OH < CH3CH2CHO < CH3COOH
Markscheme
B
Examiners report
Which molecule is polar?
A. BeCl2
B. BCl3
C. NCl3
D. CCl4
Markscheme
C
Examiners report
Which combination correctly describes the geometry of the carbonate ion, ?
Markscheme
C
Examiners report
Identifying correct electron domain and molecular geometries around a central atom was somewhat challenging and answered slighter better by higher scoring candidates.
Between which pair of molecules can hydrogen bonding occur?
A. CH4 and H2O
B. CH3OCH3 and CF4
C. CH4 and HF
D. CH3OH and H2O
Markscheme
D
Examiners report
Which pair of molecules has the same bond angles?
A. PCl3 and BCl3
B. SO2 and CO2
C. H2O and NH3
D. CCl4 and SiH4
Markscheme
D
Examiners report
Which correctly states the strongest intermolecular forces in the compounds below?
Markscheme
B
Examiners report
Which statement best describes the intramolecular bonding in HCN (l)?
A. Electrostatic attractions between H+ and CN− ions
B. Hydrogen bonding
C. Van der Waals forces and hydrogen bonding
D. Electrostatic attractions between pairs of electrons and positively charged nuclei
Markscheme
D
Examiners report
The majority of the candidates thought the bonding in HCN is ionic (electrostatic attraction between H+ and CN- ions) rather than covalent. The description of covalent bonding was the second most commonly chosen response.
Which form of carbon is the poorest electrical conductor?
A. Graphite
B. Graphene
C. Diamond
D. Carbon nanotube
Markscheme
C
Examiners report
What is the molecular geometry and bond angle in the molecular ion NO3−?
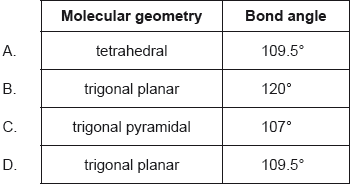
Markscheme
B
Examiners report
Which compound has the highest boiling point?
A. CH3CHO
B. CH3CH2F
C. CH3OCH3
D. CH3CH2NH2
Markscheme
D
Examiners report
How does a lithium atom form the most stable ion?
A. The atom gains a proton to form a positive ion.
B. The atom loses a proton to form a negative ion.
C. The atom loses an electron to form a positive ion.
D. The atom gains an electron to form a negative ion.
Markscheme
C
Examiners report
One of the most straight-forward questions on the paper about how lithium forms its most stable ion. It was answered correctly by 87 % of the candidates.
The electronegativity values of four elements are given.
What is the order of increasing polarity of the bonds in the following compounds?
A. CO < OF2 < NO < CF4
B. CF4 < CO < OF2 < NO
C. NO < OF2 < CO < CF4
D. CF4 < NO < OF2 < CO
Markscheme
C
Examiners report
Which substance is most likely to be ionic?
Markscheme
D
Examiners report
Which is the correct order based on increasing strength?
A. covalent bonds < hydrogen bonds < dipole–dipole forces < dispersion forces
B. dipole–dipole forces < dispersion forces < hydrogen bonds < covalent bonds
C. dispersion forces < dipole–dipole forces < hydrogen bonds < covalent bonds
D. dispersion forces < dipole–dipole forces < covalent bonds < hydrogen bonds
Markscheme
C
Examiners report
Which compound contains both ionic and covalent bonds?
A.
B.
C.
D.
Markscheme
D
Examiners report
The Lewis structure of methylamine is shown.
What is the molecular geometry around N?
A. Square planar
B. Tetrahedral
C. Trigonal planar
D. Trigonal pyramidal
Markscheme
D
Examiners report
Which molecule contains an incomplete octet of electrons?
A. NF3
B. BF3
C. BrF
D. SF2
Markscheme
B
Examiners report
66 % of the candidates identified BF3 as having an incomplete octet of electrons. The distractors NF3 and BrF were chosen in equal numbers, and the distractor SF2 was chosen by the least number of candidates.
What is the explanation for the high melting point of sodium chloride?
A. The covalent bond between sodium and chlorine atoms is strong.
B. Electrostatic attraction between sodium and chloride ions is strong.
C. Intermolecular forces in sodium chloride are strong.
D. Delocalized electrons cause strong bonding in sodium chloride.
Markscheme
B
Examiners report
Which formula is correct?
A.
B.
C.
D.
Markscheme
C
Examiners report
64% of candidates picked the correct formula for ammonium phosphate with (NH4)2PO4 being the incorrect answer chosen most frequently. NH4PO4 or (NH4)3(PO4)2 was rarely selected.
Which describes a resonance structure?
A. Double bond can be drawn in alternative positions.
B. Bonds vibrate by absorbing IR radiation.
C. A double and a single bond in the molecule
D. A Lewis structure
Markscheme
A
Examiners report
What is the type of bonding in a compound that has high boiling and melting points, poor electrical conductivity, and low solubility in water?
A. Ionic
B. Molecular covalent
C. Metallic
D. Giant covalent
Markscheme
D
Examiners report
58% of the candidates identified giant covalent bonding as the bonding in compounds with high melting and boiling points, poor electrical conductivity and low solubility in water. The most commonly selected distractor was ionic bonding. The question discriminated well between high-scoring and low-scoring candidates.
Which species does not have resonance structures?
A. C6H6
B. NH4+
C. CO32−
D. O3
Markscheme
B
Examiners report
Identifying resonance structure had one of the highest discriminatory indices. Only the higher achieving candidates had much success with this.
Which species has the same molecular geometry as SO32−?
A. BF3
B. SO3
C. PF3
D. CO32−
Markscheme
C
Examiners report
Which species has the longest carbon to oxygen bond length?
A. CO
B. CH3OH
C. CH3CO2−
D. H2CO
Markscheme
B
Examiners report
Which compound has the shortest C to N bond?
A. HCN
B. CH3CH2NH2
C. CH3CHNH
D. (CH3)2NH
Markscheme
A
Examiners report
Along which series is the bond angle increasing?
A. NH3 H2O CH4
B. CH4 NH3 H2O
C. H2O NH3 CH4
D. H2O CH4 NH3
Markscheme
C
Examiners report
Which compound has the greatest volatility under the same conditions?
A. SO2
B. SiO2
C. SnO2
D. SrO
Markscheme
A
Examiners report
For which species can resonance structures be drawn?
A. HCOOH
B. HCOO–
C. CH3OH
D. H2CO3
Markscheme
B
Examiners report
Which of the following series shows increasing hydrogen bonding with water?
A. Propane < propanal < propanol < propanoic acid
B. Propane < propanol < propanal < propanoic acid
C. Propanal < propane < propanoic acid < propanol
D. Propanoic acid < propanol < propanal < propane
Markscheme
A
Examiners report
What is the name of the compound with formula Ti3(PO4)2?
A. Titanium phosphate
B. Titanium(II) phosphate
C. Titanium(III) phosphate
D. Titanium(IV) phosphate
Markscheme
B
Examiners report
71% of candidates were able to name Ti3(PO4)2 correctly. The most commonly chosen distractor was titanium(III) phosphate.